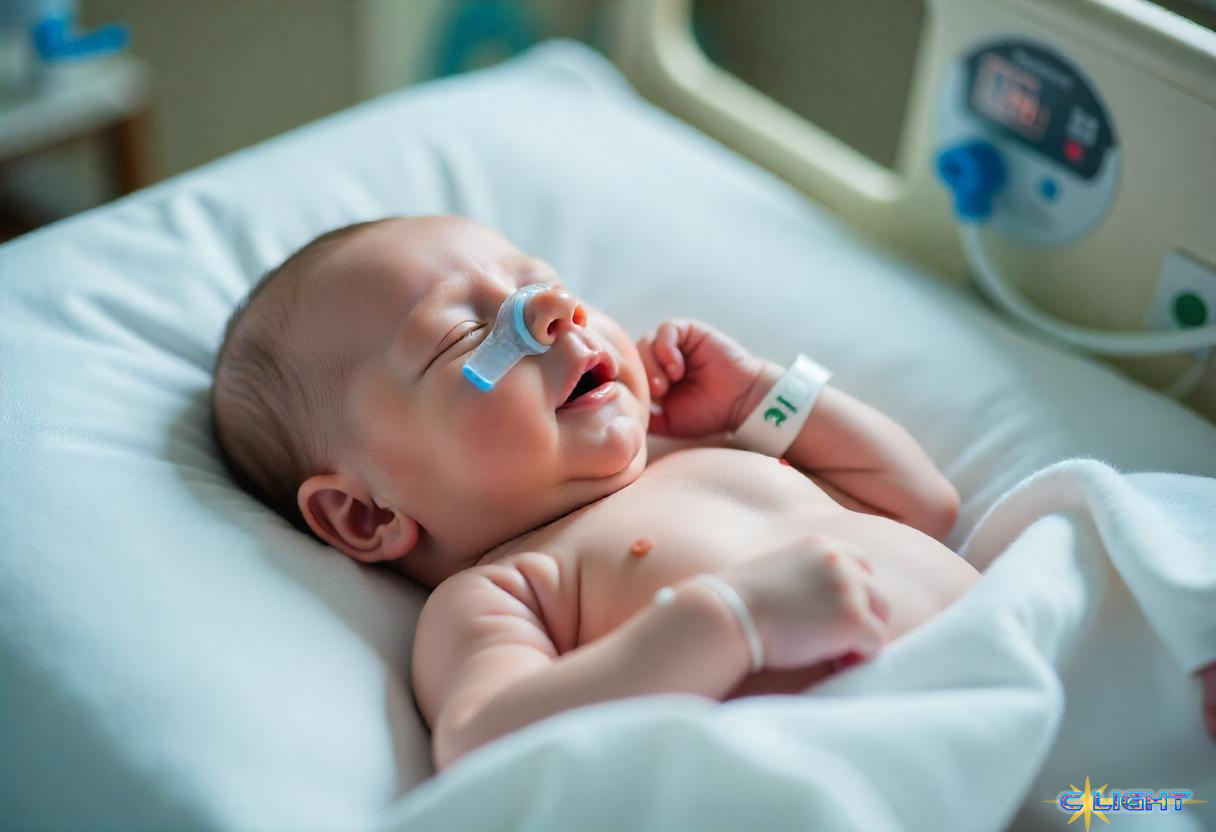
In the often-daunting world of medical headlines, a story emerged this past week from Philadelphia that radiates an almost incandescent hope. It’s the story of KJ Muldoon, an infant born in August 2024 with an ultra-rare and life-threatening genetic disorder, carbamoyl-phosphate synthetase 1 (CPS 1) deficiency, which causes toxic ammonia to ravage the body. For KJ, the prognosis was grim. Yet, in a remarkable six-month sprint, a dedicated team of scientists and doctors harnessed the revolutionary power of CRISPR gene-editing technology to create a personalized medicine, a single dose designed to correct the very “misspelling” in his genes that caused his illness. As reported in the New England Journal of Medicine and detailed in The Washington Post, young KJ, after receiving this bespoke therapy, is now nearly ten months old, meeting developmental milestones, and offering a tangible glimpse into a new era of medicine.
This isn’t just science fiction; it’s the dawn of an age where genetic diseases might be corrected at their very root. Technologies like CRISPR, particularly advanced forms like “base editing,” act like molecular scissors with extraordinary precision, allowing scientists to go into an individual’s DNA and fix the specific errors that cause inherited conditions. For KJ Muldoon, this meant a therapy developed, in all likelihood, just for him – a truly “N-of-1” medicine.
While KJ’s story is a powerful testament to treating devastating rare diseases, the horizon of gene editing’s promise extends even further, into the realm of prevention. And incredibly, that horizon is taking shape right here in our own community. During a recent visit to my oncologist here in Indianapolis just last Friday, the usual landscape of the cancer center was transformed. A significant portion of the front parking lot was a hive of construction. When I asked my doctor, “What are they building out front?” her answer was nothing short of breathtaking. She explained they are building a new stem cell and gene editing research center, its mission is to explore how these emerging technologies can be combined to potentially prevent the development of cancer in genetically predisposed babies.

The words almost don’t do justice to the scale of that ambition. It’s one thing to encounter cancer when you’re older; perhaps we’ve made some questionable choices, and our bodies, after decades, naturally begin to show wear. But to witness children, from infants to young teens, bravely facing this illness in those same hospital halls – that, as any parent or compassionate human being knows, tears the heart out. The prospect that, right here in Indianapolis, dedicated researchers will be working to spare the next generation from knowing the sheer quantity of cancer we see today is an idea so profound it “absolutely blows my mind,” as I later reflected. Suddenly, losing a few parking spaces feels like an infinitesimally small price to pay for such monumental hope.
This local endeavor, and the global efforts it mirrors, are possible only because of what former NIH Director Francis Collins, commenting on KJ’s case, called “decades of basic science research, fueled in large part by federal investments.” The “breathtaking campaign to save the life of this baby boy,” Collins noted, “would never have been possible without strong research universities and decades of government support of fundamental and translational research by NIH and FDA.” It’s a timely reminder of the value of sustained investment in science, especially as such funding faces challenges.
Of course, this new frontier is not without its hurdles. As bioethicists like Lynn Wein Bush and Henry Greely have pointed out, developing these N-of-1 therapies for rare diseases doesn’t fit traditional drug development paradigms. The costs are immense, and questions of equitable access and scalability for these highly personalized treatments are critical and still largely unanswered. Long-term safety and efficacy for any new gene-editing therapy require years of careful follow-up. And the ethical considerations surrounding any technology that alters human genes must always be navigated with profound caution and broad societal discussion, ensuring, as Bush noted, that the field doesn’t “jump ahead faster than I would ever recommend.” The team behind KJ’s therapy, acutely aware of these responsibilities, proceeded with immense care, knowing there were no guarantees for the first infant to receive such a treatment.

Yet, even with these valid cautions, the trajectory is undeniably one of immense promise. The story of KJ Muldoon, thriving against the odds, and the news of local institutions like our Indianapolis cancer center dedicating resources to the preventative power of gene editing are not isolated events. They are harbingers of a medical revolution.
As Dr. Fyodor Urnov, a scientific director at the Innovative Genomics Institute who was part of KJ’s team, expressed, “I haven’t felt this good about science in a very long time.” That sentiment is likely shared by many who see in these advancements the potential to alleviate so much human suffering.
The journey will be long, and the challenges many. But the possibility of a future where a genetic predisposition to a devastating illness is not a life sentence, where diseases like certain childhood cancers could be prevented before they even begin, is a future worth every ounce of our collective investment, research, and hope. It’s a future being built, piece by piece, by dedicated scientists, courageous families, and forward-thinking institutions, from world-renowned hospitals to the new research wing taking shape right here at home.
Discover more from Clight Morning Analysis
Subscribe to get the latest posts sent to your email.